All the services you expect from a clinical trial lab
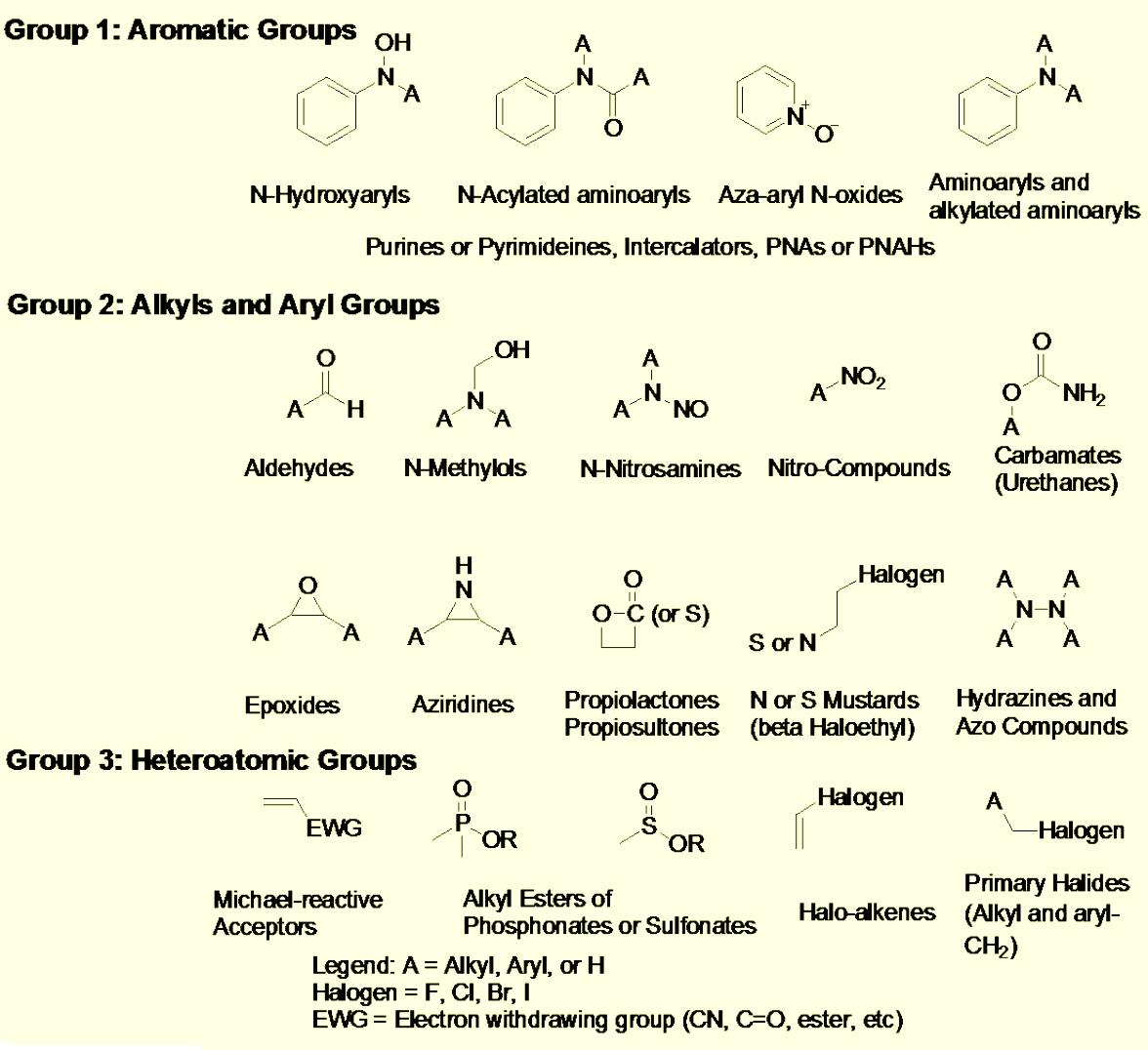
Various regulatory agencies in all over the world, require that harmful impurities in drug products be controlled and removed to below regulated limits. Genotoxins are a challenging class of impurities that have proven to be harmful even at low concentrations and as a result regulatory bodies have specifically defined their limits in drug substances and products.
The characterization and quantification of genotoxic impurities at the regulated level puts significant demands on analytical instrumentation. These requirements put forward the challenge to develop analytical procedures for different types of genotoxic impurities in different sample matrices.
With our experience and state-of -the-art instrumentation, we have the capability to develop and validate methods of genotoxic impurities in various sample matrices.
Lab technicians
Research Center
Get Social